Protocol for Making a 4% Formaldehyde Solution in PBS
The vast majority of IHC/ICC procedures employ fixation of tissues and cells using formaldehyde-based fixatives. The protocol below describes the technique for generating a 4% formaldehyde solution in PBS. The most effective fixative must be determined experimentally.
CAUTION:Formaldehyde is toxic. Please read the MSDS before working with this chemical. Gloves and safety glasses should be worn and solutions made inside a fume hood.
Please read the protocol in its entirety before starting.
Reagents Required
- Deionized H2O
- HCl (Dilute)
- NaOH (1 N)
- Paraformaldehyde Powder
- 1X PBS: 0.137 M NaCl, 0.05 M NaH2PO4, pH 7.4
Materials
- Filter Units
- Glassware & Stir Bar (dedicated for formaldehyde solution)
- Gloves & Eye Protection
- Hot Plate with Magnetic Stirrer
- Thermometer
- Ventilated hood
Procedure
- For 1 L of 4% Formaldehyde, add 800 mL of 1X PBS to a glass beaker on a stir plate in a ventilated hood. Heat while stirring to approximately 60 °C. Take care that the solution does not boil.
- Add 40 g of paraformaldehyde powder to the heated PBS solution.
- The powder will not immediately dissolve into solution. Slowly raise the pH by adding 1 N NaOH dropwise from a pipette until the solution clears.
- Once the paraformaldehyde is dissolved, the solution should be cooled and filtered.
- Adjust the volume of the solution to 1 L with 1X PBS.
- Recheck the pH, and adjust it with small amounts of dilute HCl to approximately 6.9.
- The solution can be aliquoted and frozen or stored at 2-8 °C for up to one month.
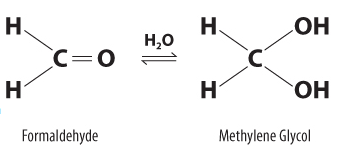
The Difference Between Paraformaldehyde, Formaldehyde, & Formalin
Paraformaldehyde (chemical name is polyoxymethylene) is a powder of polymerized formaldehyde that by itself cannot fix tissues. To be usable as a tissue fixative, paraformaldehyde has to be dissolved in hot water to become a formaldehyde solution. Formalin is a saturated formaldehyde solution in water (37% by weight, 40% by volume) containing 10-15% methanol. Methanol is added to slow down the polymerization to formaldehyde, which reduces the fixing power of formalin. Formalin can also be made in an alcohol-free form from powdered paraformaldehyde.